Kinetics and mechanistic studies on the formation and reactivity of high valent MnO porphyrin species: mono-ortho or para-substituted porphyrins versus a di-ortho-substituted one†
Abstract
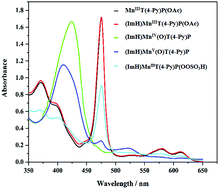
The high valent MnV(O) (λmax ≈ 407 nm) and MnIV(O) (λmax ≈ 421 nm) species of a series of electron-rich and electron-deficient meso-tetra(aryl)porphyrins (aryl = phenyl, 2-chlorophenyl, 2-nitrophenyl, 2-methylphenyl, 2-bromophenyl, 2,6-dichlorophenyl 4-methoxyphenyl, 4-methylphenyl, 4-chlorophenyl and 4-pyridyl) were prepared through the reaction of their corresponding manganese porphyrins with oxone in dichloromethane at a relatively low temperature (273 K). While the high valent Mn(O) intermediates of the para-substituted porphyrins were significantly degraded under reaction conditions, the corresponding species of the ortho substituted porphyrins showed high oxidative stability up to 20 min. Interestingly, the high valent Mn(O) of the mono-ortho-substituted meso-tetra(phenyl)porphyrins were as stable as that of meso-tetra(2,6-dichlorophenyl)porphyrin. The presence of imidazole (ImH) was found to play crucial roles in the formation and reactivity of the high valent Mn(O) species. The kinetics and mechanism of the oxidation of olefins with the high valent MnV(O) porphyrin intermediate were studied and the second order rate constants were evaluated under pseudo-first-order conditions. Also, the MnIV(O) species showed a reactivity comparable with that of the corresponding MnV(O) species. The observation of a Hammet constant of ρ = −0.48 in the oxidation of para-substituted styrenes is in accord with an electrophilic mechanism.


 Please wait while we load your content...
Please wait while we load your content...