Complexation of An(vi) with succinic acid in aqueous acid solutions: uranyl vs. plutonyl†
Abstract
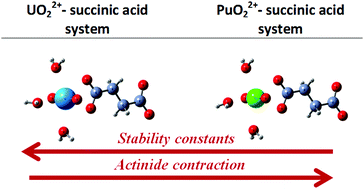
The complexation of U(VI) and Pu(VI) with succinic acid in aqueous acid solutions is investigated and compared. Affinity capillary electrophoresis (ACE) has been used to study the interaction of U(VI) with succinic acid in perchloric acid aqueous solutions (p[H] 2.0–2.5, 0.1 M ionic strength). The speciation model for uranyl is deduced in the presence of succinic acid. The existence of protonated complexes UO2HSuc+ and UO2(HSuc)2 is supposed and the stability constant values for these complex species are calculated. The interaction of Pu(VI) with succinic acid has been investigated using UV-vis spectrophotometry. The equilibria with the formation of PuO2(HSuc)2, PuO2HSuc2− and PuO2(Suc)22− complex species are found. The stability constant values are calculated at 0.1 M and 1 M ionic strengths. The existence of UO2(HSuc)2, PuO2(HSuc)2 and PuO2HSuc2− is suggested for the first time. It is noticed that the complex species of U(VI) with succinic acid are more stable than the ones of Pu(VI). The U(VI) and Pu(VI) systems with succinic acid are also investigated using density functional theory (DFT). It is observed that actinyl has a bidentate coordination with a succinate anion. Using DFT calculations the experimental finding that uranyl–succinate species are more stable than the corresponding plutonyl–succinate ones is confirmed. This trend in stability is explained by a stronger electrostatic interaction in the uranyl–succinate complexes.
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...