Triplet energy vs. electron transfers in porphyrin- and tetrabenzoporphyrin-carboxylates/Pd3(dppm)3(CO)2+ cluster assemblies; a question of negative charge†
Abstract
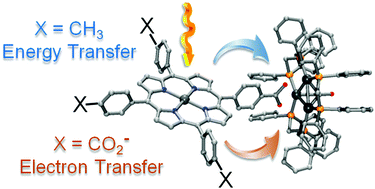
Two tetracarboxylatetetrabenzoporphyrinzinc(II), TCPBP (9,18,27,36-tetrakis-meso-(4-carboxyphenyl)tetrabenzoporphyrinatozinc(II)) and TCPEBP (9,18,27,36-tetra-(4-carboxyphenylethynyl)tetrabenzoporphyrinatozinc(II)), and their two corresponding porphyrins, TCPP (tetrakis-meso-(4-carboxyphenyl)porphyrinatozinc(II)) and TCPEP (5,10,15,20-tetra-(4-carboxyphenylethynyl)porphyrinatozinc(II)) as sodium salts in a 1 : 1 mixture of 2MeTHF/MeOH at 77 K exhibit phosphorescence in the near-IR region with maxima ranging from 785 to 1005 nm. The position of these triplet state emissions has been corroborated from DFT (B3LYP) by calculating the total energy difference between the ground, S0, and lowest energy triplet excited state T1 (the calculated position ranges from 797 to 1041 nm). At both temperatures, 77 and 298 K, these dyes make ionic driven host–guest assemblies with the unsaturated redox-active cluster Pd3(dppm)3(CO)2+ ([Pd32+], dppm = Ph2PCH2PPh2 as a PF6− salt). The formation of these assemblies is accompanied by a quenching of the phosphorescence band, without changing the emission lifetime. This phenomenon is consistent with the formation of the non-emissive assemblies dye⋯[Pd32+]x according to dye + [Pd32+] → dye⋯[Pd32+]x (x = 1–4), where only the porphyrin and tetrabenzoporphyrin dyes are phosphorescent. Quenching analysis confirmed that a static quenching mechanism operates. In one case, the quenching rate (kQ) has been evaluated from transient absorption spectroscopy (TAS) where the signal associated with the dye's triplet state exhibits a very short lifetime τ(T1), thus corroborating the efficient quenching. The quenching rate (1/τ(T1)) is much faster (3.0 × 108 s−1) than that expected for the other dye⋯[Pd32+]x assemblies in the literature (∼104 s−1) known for their triplet–triplet energy transfer. Based on the excited state driving force argumentation for oxidative quenching, this fast process is assigned to a predominant photo-induced electron transfer (3dye* + [Pd32+] → dye+˙ + [Pd3+˙]).
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...