Effect of speciation transformation of manganese on aggregation and deposition of graphene oxide†
Abstract
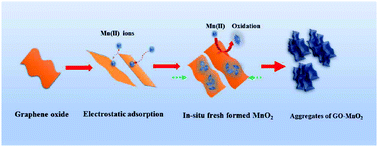
Due to the extensive existence of manganese (Mn) in natural aquatic environments and the increasing applications of graphene oxide (GO) products, the co-existence of Mn and GO in natural waters would be inevitable. However, the effect of their interactions on the transportation and fate of GO is not clear. In this work, the impact of different species of manganese on the aggregation of GO was investigated systematically through batch experiments under different chemical conditions (e.g., Mn concentration and pH). The results indicated that the concentration of Mn(II) played a significant role in the aggregation and deposition of GO. The critical coagulation concentration (CCC) of Mn(II) for the aggregation of GO suspension (within the range of 5–100 mg L−1) was about 3.0 mM at neutral pH conditions. At high pH values (8–10), only 0.3 mM Mn could destabilize the GO suspension due to the in situ freshly formed oxidized manganese species through mechanisms of electrostatic-adsorption, neutralization, and catching–sweeping interactions. However, addition of pre-prepared MnO2 had little effect on aggregating the GO suspension over a wide range of pH value. Overall, these findings are useful for understanding and predicting the fate and transport of GO in both aquatic environments and wastewater treatment areas in the presence of manganese.


 Please wait while we load your content...
Please wait while we load your content...