Fluorescent biogenic Schiff base compounds of dimethyltin†
Abstract
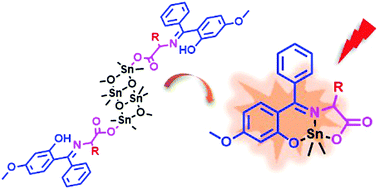
Herein, biologically crucial amino acids have been explored for the synthesis of fluorescent organotin compounds. One-pot reaction of dimethyltin oxide, 2-hydroxy-4-methoxybenzophenone and α-amino acids, i.e., glycine, L-isoleucine and L-methionine, afforded respective organotin compounds with biogenic Schiff base skeletons 1–3. Although all the compounds were constituted from similar ONO type Schiff base units, only compound 1 (derived from glycine) crystallized as a hexacoordinated organotin compound of the type (ONO)SnMe2(MeOH) with a tridentate chelating Schiff base ligand. Analogous syntheses with L-isoleucine and L-methionine did not afford the related Schiff base compounds 2 and 3. Instead, some kind of precursors, namely 2′ and 3′, crystallized as solvent mediated Sn4O4 ladders and two Schiff base ligand moieties bound to the terminal Sn atoms via carboxylate moiety only. Interestingly, solution NMR spectroscopy (1H, 13C, 119Sn) of 1 unambiguously complied with its solid state structure while for 2′ and 3′ the behaviour was entirely different suggesting their transformation to mononuclear compounds 2 and 3 in solution phase. The distinctive structural aspects of 2′/2 and 3′/3 in solid and solution phase were also supported by solid state 119Sn CP/MAS NMR studies. All the compounds were found to be fluorescent when excited at 365 nm and the solutions of compound 3′ experienced quenching in the presence of paramagnetic metal ions due to chelation enhanced quenching.


 Please wait while we load your content...
Please wait while we load your content...