New coordination polymers based on 2-methylimidazole and transition metal nitroprusside containing building blocks: synthesis, structure and magnetic properties†
Abstract
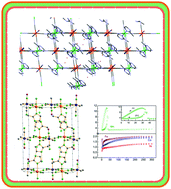
Four coordination polymers obtained by the intercalation of 2-methylimidazole into transition metal nitroprussides T[Fe(CN)5NO] with T = Mn, Fe, Co and Ni were synthesized and characterized. The resulting hybrid inorganic–organic solids crystallize as dihydrates. All the solids were crystallized in an orthorhombic crystal system in the space group P212121. Their crystal structures were solved and refined from X-ray diffraction data, complemented by structural information derived from spectroscopic (IR, Raman and UV-Vis) and thermal data. The intercalated molecules occupy the axial coordination sites of the metal T(II), while the Fe atom of the nitroprusside anion preserves the octahedral coordination geometry. In the interlayer region, the intercalated molecules retain their interaction through π-stacking interactions. The magnetic measurements indicate that all the solids show antiferromagnetic interactions at low temperatures due to intra-sheet antiferromagnetic exchange interactions of moderate strength. Only complexes with Fe(II) and Mn(II) show long-range inter-sheet ferromagnetic ordering.


 Please wait while we load your content...
Please wait while we load your content...