Synthesis, characterization, and antiproliferative and apoptosis inducing effects of novel s-triazine derivatives†
Abstract
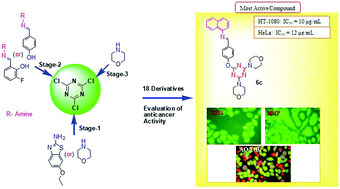
In an attempt to design and synthesize a new class of antitumor agents, a mild and eco-friendly protocol for nucleophilic substitution using an s-triazine scaffold, via amine and Schiff base derivatives, has been developed. In order to obtain antitumor activity, all synthesized compounds were screened in vitro for their cytotoxicity against human fibrosarcoma tumor cells (HT-1080) and a cervical cancer cell line (HeLa), for their ability to inhibit the growth of cancer cells. The selected s-triazine analogs (5c, 5d, and 6c) have been preliminarily studied for their reactive oxygen species (ROS) properties, mitochondrial membrane potential (MMP) and apoptosis (AO/EtBr) activity against the HT-1080 cancer cell line. The in vitro anticancer activity analysis has revealed that the synthesized compounds have good/moderate inhibitory activity against the tested cell lines compared to the standard drug. The theoretical study results also provide evidence that the s-triazines scaffolds have been successfully identified as superior p53-MDM2 inhibitors through structure-based design.


 Please wait while we load your content...
Please wait while we load your content...