Adsorption of diclofenac on a magnetic adsorbent based on maghemite: experimental and theoretical studies†
Abstract
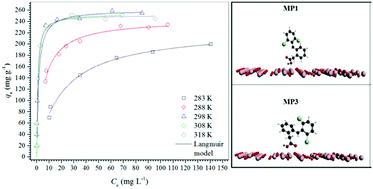
In the present study, a magnetic adsorbent for diclofenac formed by maghemite (γ-Fe2O3) nanoparticles with high saturation magnetization (19.8 emu g−1) and specific area (79 m2 g−1) was synthesized by a one-pot method through the precipitation of Fe2+ ions with NaOH solution followed by rapid oxidation with hydrogen peroxide. The X-ray diffraction and Mössbauer spectroscopy data confirmed that the adsorbent is formed solely by maghemite. The adsorption equilibrium time for diclofenac (C0 = 500 mg L−1) was reached after 120 min, and the kinetic data were best fitted to the pseudo-first-order model. The adsorption isotherms acquired at five different temperatures showed an increase in the maximum adsorption capacity (261 mg g−1) until 298 K, but at higher temperatures, the maximum adsorption capacity was not increased. The isotherm data were best fitted to the Langmuir and Sips models. Adsorption tests as a function of solution pH showed a decrease in the diclofenac adsorption capacity with increasing solution pH, suggesting that the hydroxyl anions compete with diclofenac molecules for the adsorption sites. Diclofenac adsorption on maghemite was endothermic (67.31 kJ mol−1) and entropically driven (TΔadsS° = 96.33 kJ mol−1). Finally, theoretical calculations and infrared spectroscopy data suggest a physisorption mechanism of diclofenac on the maghemite surface.


 Please wait while we load your content...
Please wait while we load your content...