Ligand-assisted reduction and reprecipitation synthesis of highly luminescent metal nanoclusters†
Abstract
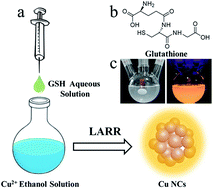
Solid-state luminescent materials play a key role in fabricating light-emitting diodes (LEDs). Herein, highly luminescent metal nanoclusters (NCs) are synthesized using a ligand-assisted reduction and reprecipitation process. Glutathione (GSH) dissolved in a good solvent (water) is injected into a poor solvent (ethanol in which Cu2+ is dissolved), where the fast reduction of Cu2+ by GSH and the supersaturation-induced aggregation triggered by the solubility change of GSH upon solvent mixing occur. Nanoparticles with diameters of around 50–80 nm embedded with small-sized Cu NCs (around 2 nm) can be obtained and processed into powders simply by drying the solvent. The powders show bright-orange emission with a photoluminescence quantum yield as high as 48%. Nearly monoexponential behavior was observed in the photoluminescence decay profiles of the Cu NCs, which can be attributed to the abundance of metal defect-related states formed with the assistance of coordination between Cu and ethanol. Moreover, white LEDs were fabricated using blue-emissive commercial phosphors and orange-emissive Cu NCs as color converters integrated with UV LED chips.


 Please wait while we load your content...
Please wait while we load your content...