Pneumococcal histidine triads – involved not only in Zn2+, but also Ni2+ binding?†
Abstract
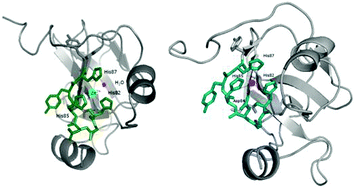
Polyhistidine triad proteins, which participate in Zn2+ uptake in Streptococcus pneumoniae, contain multiple copies of the HxxHxH (histidine triad motif) sequence. We focus on three such motifs from one of the most common and well-conserved polyhistidine triad proteins, PhtA, in order to understand their bioinorganic chemistry; particular focus is given to (i) understanding which of the PhtA triads binds Zn2+ with the highest affinity (and why) and (ii) explaining whether Ni2+ (also crucial for bacterial survival and virulence) could potentially outcompete Zn2+ at its native binding site. There is no significant difference in the stability of zinc(II) complexes with the three studied protein fragments, but one of the nickel(II)–polyhistidine triads is remarkably stable; we explain why and hypothesize about the biological importance of this finding.
- This article is part of the themed collection: In memory of Silvia Atrian


 Please wait while we load your content...
Please wait while we load your content...