Calprotectin influences the aggregation of metal-free and metal-bound amyloid-β by direct interaction†
Abstract
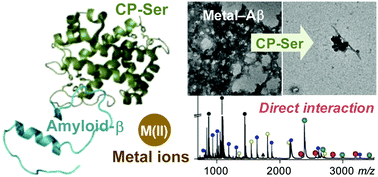
Proteins from the S100 family perform numerous functions and may contribute to Alzheimer's disease (AD). Herein, we report the effects of S100A8/S100A9 heterooligomer calprotectin (CP) and the S100B homodimer on metal-free and metal-bound amyloid-β (Aβ; Aβ40 and Aβ42) aggregation in vitro. Studies performed with CP-Ser [S100A8(C42S)/S100A9(C3S) oligomer] indicate that the protein influences the aggregation profile for Aβ40 in both the absence and presence of metal ions [i.e., Zn(II) and Cu(II)]. Moreover, the detection of Aβ40–CP-Ser complexes by mass spectrometry suggests a direct interaction as a possible mechanism for the involvement of CP in Aβ40 aggregation. Although the interaction of CP-Ser with Aβ40 impacts Aβ40 aggregation in vitro, the protein does not attenuate Aβ-induced toxicity in SH-SY5Y cells. In contrast, S100B has a slight effect on the aggregation of Aβ. Overall, this work supports a potential association of CP with Aβ in the absence and presence of metal ions in AD.


 Please wait while we load your content...
Please wait while we load your content...
