Spatially resolved solid-state reduction of graphene oxide thin films†
Abstract
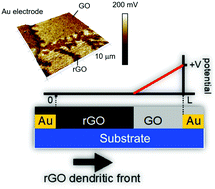
Re-establishment of electrical conductivity in graphene oxide (GO), the insulating form of graphene, is (partially) accomplished by reduction through high temperature treatments in a reducing atmosphere, or using strongly reducing chemicals or electrolytic processes. The reduction methods are suited for bulk graphene oxide. Spatially resolved reduction of thin films of graphene oxide is important for a wide range of applications such as in microelectronics, where an electrolyte-free, room temperature reduction process is needed. Here, we present spatially resolved solid-state reduction of graphene oxide thin films. We demonstrate that the reduction mechanism is based on electrolysis of water that is adsorbed on the graphene oxide thin film. The reduced graphene oxide thin-films show sheet resistance of only several kOhm, with weak temperature dependence. Graphene oxide can be produced on a large scale and processed using low-cost solution casting techniques. Spatially resolved re-establishment of conductivity in GO can be used in electrically controlled water permeation or in micro- and nanoelectronic applications for instance as an anti-fuse.
- This article is part of the themed collection: International Year of the Periodic Table : Low Dimensional Carbon Systems

 Please wait while we load your content...
Please wait while we load your content...