Tailoring of carbon nanotubes for the adsorption of heavy metal ions: molecular dynamics and experimental investigations†
Abstract
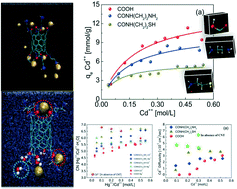
Polluted water sources due to enormously enhanced human population or rapid industrialization need to be treated as they have an adverse effect on human health. CNTs are becoming quite popular in the field of adsorption, which on functionalization with various functional groups can be employed as effective adsorbents. The present study explores the adsorption mechanism of Cd++/Hg++ with carboxyl (COOH), amine (CONH(CH2)2NH2) and sulfur (CONH(CH2)2SH) based CNTs, using molecular dynamics (MD) simulations. The study investigates the molecular level events of adsorption both in aqueous and acidic environments. The MD studies capture well the experimentally observed Langmuir type adsorption isotherms of metal ions for the CNTs. The presence of acid was found to reduce the adsorption of metal ions due to competition with H3O+ ions. The results establish the use of amine based CNTs over sulfur based CNTs for the adsorption of Cd++ ions. However, for the adsorption of Hg++, both amine based CNTs as well as sulfur based CNTs were found to be equally suitable. The simulation trajectories visualize the binding sites for adsorbed ions, which preferentially reside near the O atom for most of the functional groups and also near the N atom of the amine at higher concentration. In addition, the adsorption correlation function and corresponding residence time distribution were used to determine the strength of binding. Also, the rich structural and dynamical events were explored via RDF, CNs and diffusion coefficients. The higher the adsorption capacity of the functionalized CNTs, the lower the hydration number of the metal ions was estimated compared to the bulk value. In particular, the dynamics of the metal ions were found to support well the trend, followed by the primary hydration number as well as the order of the maximum adsorption capacity (qmax) exhibited by the different CNTs. The present study provides insights and quantitative information for the adsorption of metal ions with functionalized CNTs, which might be very useful for the exploration of the myriads of nano-adsorbent based experiments and thus future technology.


 Please wait while we load your content...
Please wait while we load your content...