Design, synthesis, and biological activity of novel ammonium salts containing sterically hindered phenolic fragment and phosphoryl group†
Abstract
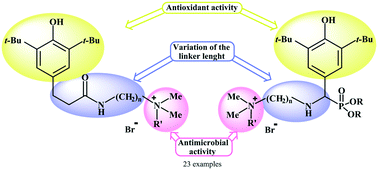
Here, we present an approach to novel “hybrid” biologically active compounds based on a combination of sterically hindered phenol and ammonium pharmacophores in a single molecule. The novel target ammonium salts were obtained by the reaction of 3-(3,5-di-tert-butyl-4-hydroxyphenyl)-N-(2-(dimethylamino)alkyl)propanamide with aliphatic bromides or by the reaction of phosphorylated methylenequinones with diamines followed by alkylation with organic bromides. A series of twenty-three novel multifunctional ammonium salts that contain a sterically hindered phenolic fragment were assessed for antimicrobial, cytotoxic and antioxidant activity. The compounds exhibited antimicrobial activity against Staphylococcus aureus ATCC 209p, Bacillus cereus ATCC 8035, Escherichia coli CDC F-50, Pseudomonas aeruginosa ATCC 9027, Aspergillus niger BKMF-1119, Trichophyton mentagrophytes var. gypseum 1773, and Candida albicans 855-653 in the concentration range of 442–0.70 μM. The maximum activity of an ammonium salt among all the types of structure was observed in cases in which a decyl radical was present on the onium nitrogen atom. The most active compounds exhibited antioxidant activity at levels of 0.25 and 0.50 mM and did not display cytotoxic properties towards WI-38 (human embryonic lung cells) and Chang liver (human liver cells) cell lines in the concentration range of 0.70–11.3 μM.


 Please wait while we load your content...
Please wait while we load your content...