Exploring the effectiveness of novel benzimidazoles as CB2 ligands: synthesis, biological evaluation, molecular docking studies and ADMET prediction†
Abstract
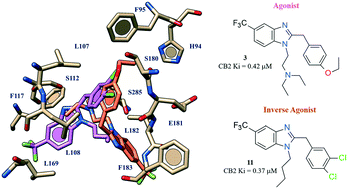
Herein we continued our previous work on the development of CB2 ligands, reporting the design and synthesis of a series of benzimidazole-containing derivatives that were explored as selective CB2 ligands with binding affinity towards both CB1 and CB2 receptors. Seven out of eighteen compounds exhibited preferential binding ability to CB2 over CB1 receptors with potencies in the sub-micromolar or low micromolar range. In particular, we identified two promising hit compounds, the agonist 1-[2-(N,N-diethylamino)ethyl]-2-(4-ethoxybenzyl)-5-trifluoromethylbenzimidazole (3) (CB2: Ki = 0.42 μM) and the inverse agonist/antagonist 1-butyl-2-(3,4-dichlorobenzyl)-5-trifluoromethylbenzimidazole (11) (CB2: Ki = 0.37 μM). Docking studies also performed on other benzimidazoles reported in the literature supported the structure–activity relationship observed in this series of compounds and allowed the key contacts involved in the agonist and/or inverse agonist behaviour displayed by these derivatives to be determined. The in silico evaluation of ADMET properties suggested a favorable pharmacokinetic and safety profile, promoting the drug-likeness of these compounds towards a further optimization process.
- This article is part of the themed collection: Molecular Pharmacology


 Please wait while we load your content...
Please wait while we load your content...