Novel O-methyl goniofufurone and 7-epi-goniofufurone derivatives: synthesis, in vitro cytotoxicity and SAR analysis†
Abstract
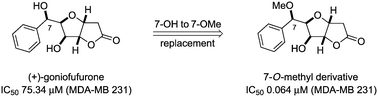
Novel goniofufurone (1) and 7-epi-goniofufurone (2) derivatives bearing a methoxy group at the C-5 and/or C-7 positions were prepared and their in vitro antitumour activity against some human tumour cell lines was evaluated. Some of the analogues displayed powerful antiproliferative effects against the studied tumour cells, but almost all of them were non-cytotoxic toward the normal cells (MRC-5). A SAR study reveals that the introduction of a methoxy group at the C-7 position may increase the antiproliferative effects of the analogues. The most active compounds are 7-O-methyl derivatives of goniofufurone (3) and 7-epi-(+)-goniofufurone (6), which exhibited 1177- and 451-fold higher potencies than the leads 1 and 2 toward the MDA-MB 231 cell line. At the same time, compound 3 is almost 1.5-fold more active than the commercial drug doxorubicin (DOX) against the same cell line. Flow cytometry data confirmed that the cytotoxic effects of these analogues are mediated by apoptosis, additionally revealing that these molecules induced changes in the K562 cell cycle distribution.


 Please wait while we load your content...
Please wait while we load your content...