Design, synthesis, and in vitro biological evaluation of novel benzimidazole tethered allylidenehydrazinylmethylthiazole derivatives as potent inhibitors of Mycobacterium tuberculosis†
Abstract
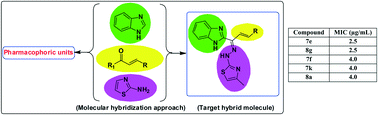
Tuberculosis (TB) has become one of the most significant public health problems in recent years. Antibiotic therapy remains the mainstay of TB control strategies, but the increasing resistance of mycobacterial species has heightened alarm, requiring the development of novel drugs in order to improve treatment outcomes. Here, as an effort to identify novel and effective antitubercular agents, we designed and synthesized a series of novel substituted benzimidazolallylidenehydrazinylmethylthiazole derivatives via a multi-component molecular hybridization approach with single molecular architecture. Our design strategy involved assembling the antitubercular pharmacophoric fragments benzimidazole, 2-aminothiazole and substituted α,β-unsaturated ketones via condensation reactions. All the newly synthesized compounds were fully characterized via NMR and mass spectral data and evaluated for in vitro biological activity against the H37Ra strain of Mycobacterium tuberculosis. From the biological evaluation data, we identified some effective compounds, of which 8g and 7e were the most active ones (both having MIC values of 2.5 μg mL−1). In addition, compound 8g exhibited a lower cytotoxicity profile. We conceive that compound 8g may serve as a chemical probe of interest for further lead optimization studies with the general aim of developing novel and effective antitubercular agents.


 Please wait while we load your content...
Please wait while we load your content...