Novel hybrids derived from aspirin and chalcones potently suppress colorectal cancer in vitro and in vivo†
Abstract
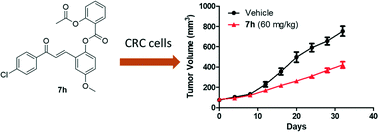
Colorectal cancer (CRC) remains the fourth leading cause of cancer deaths around the world despite the availability of many approved small molecules for treatment. The issues lie in the potency, selectivity and targeting of these compounds. Therefore, new strategies and targets are needed to optimize and develop novel treatments for CRC. Here, a group of novel hybrids derived from aspirin and chalcones were designed and synthesized based on recent reports of their individual benefits to CRC targeting and selectivity. The most active compound 7h inhibited proliferation of CRC cell lines with better potency compared to 5-fluorouracil, a currently used therapeutic agent for CRC. Importantly, 7h had 8-fold less inhibitory activity against non-cancer CCD841 cells. In addition, 7h inhibited CRC growth via the inhibition of the cell cycle in the G1 phase. Furthermore, 7h induced apoptosis by activating caspase 3 and PARP cleavage, as well as increasing ROS in CRC cells. Finally, 7h significantly retarded the CRC cell growth in a mouse xenograft model. These findings suggest that 7h may have potential to treat CRC.


 Please wait while we load your content...
Please wait while we load your content...