Development of subnanomolar-affinity serotonin 5-HT4 receptor ligands based on quinoline structures†
Abstract
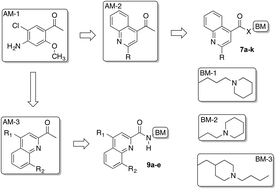
Two small series of quinoline derivatives were designed starting from previously published quinoline derivatives 7a and b in order to obtain information about their interaction with the 5-HT4R binding site. Initially, the structure of 7a and b was modified by replacing their basic moiety with that of partial agonist 4 (ML10302) or with that of reference ligand 6 (RS-67-333). Then, the aromatic moieties of 4-quinolinecarboxylates 7a, d–f, and h–k or 4-quinolinecarboxamides 7b, c, and g were modified into those of 2-quinolinecarboxamides 9a–e. Very interestingly, this structure–affinity relationship study led to the discovery of 7h–j as novel 5-HT4R ligands showing Ki values in the subnanomolar range. The structures of all these compounds contain the N-butyl-4-piperidinylmethyl substituent, which appear to behave as an optimized basic moiety in the interaction of these 4-quinolinecarboxylates with the 5-HT4R binding site. However, this basic moiety was ineffective in providing 5-HT4R affinity in the corresponding 4-quinolinecarboxamide 7g, but it did in 2-quinolinecarboxamide ligands 9c–e. Thus, a subtle interrelationship of several structural parameters appeared to play a major role in the interaction of the ligands with the 5-HT4R binding site. They include the kind of basic moiety, the position of the carbonyl linking group with respect to the aromatic moiety and its orientation, which could be affected by the presence of the intramolecular H-bond as in compounds 9c–e.


 Please wait while we load your content...
Please wait while we load your content...