Curcumin-based pyrazoline analogues as selective inhibitors of human monoamine oxidase A†
Abstract
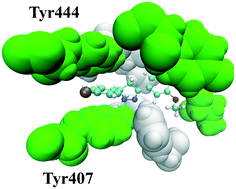
A series of 2-methoxy-4-(5-phenyl-4,5-dihydro-1H-pyrazol-3-yl)phenol (pyrazoline) derivatives (2–6) have been synthesized and tested for human monoamine oxidase (hMAO) inhibitory activity. The most active derivative (2) behaved as a competitive hMAO-A inhibitor, with an inhibition constant value of 0.08 μM and a strong hMAO-A selectivity (Ki(hMAO-B)/Ki(hMAO-A) > 1751). In addition, 2 exhibited little to no cytotoxic effects up to a 25 μM concentration and provided the best blood–brain barrier permeability among the derivatives synthesized. Molecular dynamics simulations revealed that a chlorine substituent at the para-position of the phenyl ring in 2 enabled a π–π stacking interaction with Tyr407 and Tyr444 that resulted in the formation of an “aromatic sandwich” structure. Consequently, this tight-binding aromatic cage culminated in a dramatically reduced active site volume that is believed to be the origin of the observed selectivity between the hMAO-A and hMAO-B isozymes. Removal of the chlorine from 2 disrupted the favorable intermolecular interactions and resulted in a selectivity change towards hMAO-B.


 Please wait while we load your content...
Please wait while we load your content...