Efficient copper-catalyzed amination of DNA-conjugated aryl iodides under mild aqueous conditions†
Abstract
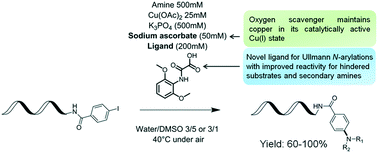
Herein, we describe the development of copper-catalyzed cross-coupling of DNA-conjugated aryl iodides with aliphatic amines. This protocol leverages a novel ligand, 2-((2,6-dimethoxyphenyl)amino)-2-oxoacetic acid, to effect the transformation in aqueous DMSO, under mild conditions and in air, making it an ideal candidate for the synthesis of DNA-encoded libraries.


 Please wait while we load your content...
Please wait while we load your content...