Tricyclic xanthine derivatives containing a basic substituent: adenosine receptor affinity and drug-related properties†
Abstract
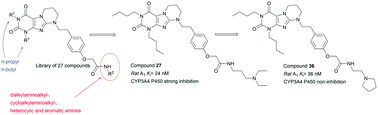
A library of 27 novel amide derivatives of annelated xanthines was designed and synthesized. The new compounds represent 1,3-dipropyl- and 1,3-dibutyl-pyrimido[2,1-f]purinedione-9-ethylphenoxy derivatives including a CH2CONH linker between the (CH2)2-amino group and the phenoxy moiety. A synthetic strategy to obtain the final products was developed involving solvent-free microwave irradiation. The new compounds were evaluated for their adenosine receptor (AR) affinities. The most potent derivatives contained a terminal tertiary amino function. Compounds with nanomolar AR affinities and at the same time high water-solubility were obtained (A1 (Ki = 24–605 nM), A2A (Ki = 242–1250 nM), A2B (Ki = 66–911 nM) and A3 (Ki = 155–1000 nM)). 2-(4-(2-(1,3-Dibutyl-2,4-dioxo-1,2,3,4,7,8-hexahydropyrimido[2,1-f]purin-9(6H)-yl)ethyl)phenoxy)-N-(3-(diethylamino)propyl)acetamide (27) and the corresponding N-(2-(pyrrolidin-1-yl)ethyl)acetamide (36) were found to be the most potent antagonists of the present series. While 27 showed CYP inhibition and moderate metabolic stability, 36 was found to possess suitable properties for in vivo applications. In an attempt to explain the affinity data for the synthesized compounds, molecular modeling and docking studies were performed using homology models of A1 and A2A adenosine receptors. The potent compound 36 was used as an example for discussion of the possible ligand–protein interactions. Moreover, the compounds showed high water-solubility indicating that the approach of introducing a basic side chain was successful for the class of generally poorly soluble AR antagonists.


 Please wait while we load your content...
Please wait while we load your content...