Phenylethynyl-substituted heterocycles inhibit cyclin D1 and induce the expression of cyclin-dependent kinase inhibitor p21Wif1/Cip1 in colorectal cancer cells
Abstract
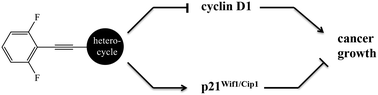
Fluorinated phenylethynyl-substituted heterocycles that possessed either an N-methylamino or N,N-dimethylamino group attached to heterocycles including pyridines, indoles, 1H-indazoles, quinolines, and isoquinolines inhibited the proliferation of LS174T colon cancer cells in which the inhibition of cyclin D1 and induction of the cyclin-dependent kinase inhibitor-1 (i.e., p21Wif1/Cip1) served as readouts for antineoplastic activity at a cellular level. At a molecular level, these agents, particularly 4-((2,6-difluorophenyl)ethynyl)-N-methylisoquinolin-1-amine and 4-((2,6-difluorophenyl)ethynyl)-N,N-dimethylisoquinolin-1-amine, bound and inhibited the catalytic subunit of methionine S-adenosyltransferase-2 (MAT2A).


 Please wait while we load your content...
Please wait while we load your content...