Micropassage-embedding composite hydrogel fibers enable quantitative evaluation of cancer cell invasion under 3D coculture conditions†
Abstract
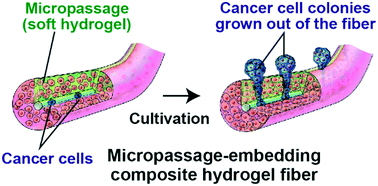
Cell migration and invasion are of significant importance in physiological phenomena, including wound healing and cancer metastasis. Here we propose a new system for quantitatively evaluating cancer cell invasion in a three-dimensional (3D), in vivo tissue-like environment. This system uses composite hydrogel microfibers whose cross section has a relatively soft micropassage region and that were prepared using a multilayered microfluidic device; cancer cells are encapsulated in the core and fibroblasts are seeded in the shell regions surrounding the core. Cancer cell proliferation is guided through the micropassage because of the physical restriction imposed by the surrounding solid shell regions. Quantitative analysis of cancer cell invasion is possible simply by counting the cancer cell colonies that form outside the fiber. This platform enables the evaluation of anticancer drug efficacy (cisplatin, paclitaxel, and 5-fluorouracil) based on the degree of invasion and the gene expression of cancer cells (A549 cells) with or without the presence of fibroblasts (NIH-3T3 cells). The presented hydrogel fiber-based migration assays could be useful for studying cell behaviors under 3D coculture conditions and for drug screening and evaluation.


 Please wait while we load your content...
Please wait while we load your content...