Insight into the mechanisms controlling the chemical vapor generation of cadmium†
Abstract
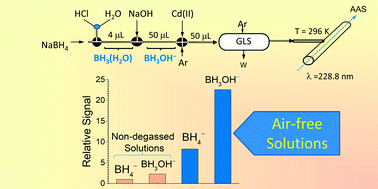
Chemical vapor generation of cadmium (CVG) by aqueous NaBH4 was investigated with the aim to individuate the mechanisms controlling the generation of volatile cadmium species in the absence of any additive. A continuous flow reactor was employed in combination with atomic absorption spectrometry. An electro-thermal quartz tube atomizer (293–1200 K) and a miniature argon–hydrogen diffusion flame were employed as the atomizers. The reactor system can be used with various chemifold setups which allow different mixing strategies of the sample (2.5–50 μg L−1 of CdII in 0.25 mol L−1 HCl) and reductant (1–3% m/v NaBH4 in 0.1–1.2 mol L−1 NaOH) solutions. The molar ratios of the reagents HCl, NaBH4 and NaOH play a decisive role in determining both the nature of CdII substrates and the hydrolysis products of BH4−, which strongly affected the generation efficiency of volatile Cd species. For the first time the interference effect of dissolved oxygen and the use of BH3OH− in CVG of Cd have been demonstrated. The interference of dissolved oxygen, causing liquid phase interference during the derivatization step, can be removed by degassing the solution with argon. The on-line generation of BH3OH− in a quenched flow reactor indicates that it is more efficient than that of BH4− and it might play a role in CVG of Cd. The combination of BH3OH− derivatization and degassed solutions allows obtaining about 20 and 5 fold increase of sensitivity using a quartz tube and miniature flame, respectively. Volatile cadmium vapors are composed of both atomic and molecular species, and the oxygen removal and/or the use of BH3OH− favors the formation of atoms with respect to molecular species.


 Please wait while we load your content...
Please wait while we load your content...