Computational redesign of penicillin acylase for cephradine synthesis with high kinetic selectivity†
Abstract
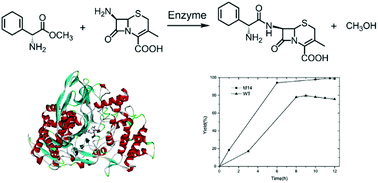
Computational redesign of native enzyme active sites for non-natural substrates offers a general approach for creating biocatalysts in green chemistry. Herein, a computational strategy was developed to discover selective mutants of penicillin acylase to catalyze the condensation reaction between D-dihydrophenylglycine methyl ester (DHME) and 7-aminodesacetoxy cephalosporanic acid (7-ADCA), producing cephradine in fully aqueous medium. The key feature of this strategy is that mutants favoring the binding of the near-attack conformation of cephradine in the catalytic orientation were computationally selected using a scoring function based on discounted folding energy relative to binding energy. Using this strategy, we obtained a penicillin acylase mutant (M142αF/F24βA/S67βA) with high kinetic selectivity that increased the synthesis/hydrolysis ratio (S/H) by more than 10-fold compared with the wild-type. In its immobilized form, the redesigned triple mutant attained up to 99% yield under industrial conditions. This study represents a breakthrough in enzymatic synthesis of cephradine and suggests that computational design strategies can adapt an enzyme to catalyze non-natural chemical transformations for green process development with industrial significance.


 Please wait while we load your content...
Please wait while we load your content...