Enzymatic reduction of levoglucosenone by an alkene reductase (OYE 2.6): a sustainable metal- and dihydrogen-free access to the bio-based solvent Cyrene®†
Abstract
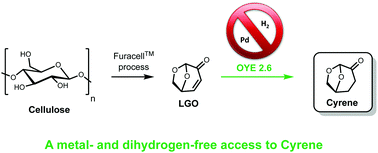
Levoglucosenone (LGO) has been successfully converted into the green polar aprotic solvent 2H-LGO (aka Cyrene®) through an enzymatic process involving alkene reductases: wild-type Old Yellow Enzyme 2.6 (OYE 2.6 wt.) from Pichia stipitis and its mutant (OYE 2.6 Tyr78Trp) present the best conversion rates. This enzymatic process has been optimized in order to avoid the formation of the side-product (1R,2S)-2-hydroxy-6,8-dioxabicyclo[3.2.1]octan-4-one (OH-LGO) and reach total conversion (99%). Cyrene® was then successfully isolated by continuous extraction in quantitative yield (99%).


 Please wait while we load your content...
Please wait while we load your content...
