Electro-reduction of hematite using water as the redox mediator†
Abstract
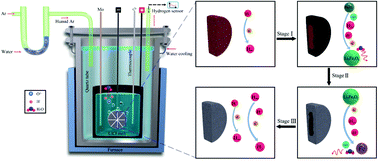
The primary production of iron from its oxides, about 1.6 billion tons per annum, alone is several times greater than the production capacity of all other metals together. This massive production is conventionally achieved by carbothermal reduction causing several million tons of greenhouse CO2 emissions every year, with catastrophic environmental consequences. Here, we present a green, efficient and economical molten salt route for the electrolytic production of iron from iron oxide (hematite, Fe2O3). The dissolution of water in molten LiCl causes the formation of hydrogen cations in the melt, which can be discharged on cathodically polarized solid iron oxide pellets immersed in the melt, leading to the reduction of Fe2O3 of metallic iron and the regeneration of water. The Fe2O3 pellet was thoroughly reduced to iron under 1.4 V within 5 h, considerably faster and more energy efficient than the current electro-deoxidation methods available. The feasibility of the water induced electrochemical reduction of metal oxides is confirmed using Fe2O3, but this novel methodology should be applicable to a broad range of metal oxides.


 Please wait while we load your content...
Please wait while we load your content...