Solvent-free mechanochemical oxidation and reduction of biomass-derived 5-hydroxymethyl furfural†
Abstract
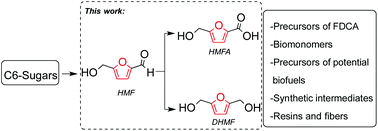
The simultaneous synthesis of 5-hydroxymethyl-2-furoic acid and 2,5-hydroxymethylfuran from biomass-derived 5-hydroxymethyl furan was developed using a solvent-free mechanochemical approach. The results obtained for the Cannizzaro disproportionation reaction show quantitative conversions of the starting materials with reaction times of only 5 min. Employing solvent-free conditions allows for a more sustainable synthetic approach that is reflected in an Efactor 7 times smaller than that in previous reports. Additionally, initial results of the use of a sacrificial reagent, with the same solvent-free mechanochemical approach, for the selective reduction and oxidation of HMF are presented.


 Please wait while we load your content...
Please wait while we load your content...