Catalytic production of hexane-1,2,5,6-tetrol from bio-renewable levoglucosanol in water: effect of metal and acid sites on (stereo)-selectivity†
Abstract
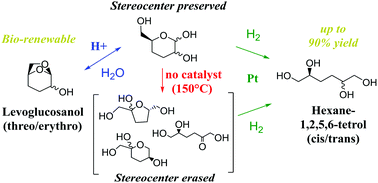
We report on a new route to produce hexane-1,2,5,6-tetrol (tetrol) from cellulose-derived levoglucosanol (lgol). We investigate the reaction intermediates formed over metal and acid catalysts, and propose a reaction network for this process. Lgol is converted to tetrol in up to 90% yield over a bifunctional Pt/SiO2–Al2O3 catalyst at 150 °C. High tetrol yields are maintained at lgol concentrations of up to 21 wt% in water. threo- and erythro-lgol first undergo hydrolysis to 3,4-dideoxymannose (DDM) and 3,4-dideoxyglucose (DDG), respectively. This reaction can be carried out selectively over an Amberlyst 70 acid catalyst at a temperature of 100 °C. At a higher temperature of 150 °C with no added catalyst, DDM and DDG undergo aldose–ketose isomerization to 3,4-dideoxyfructose (DDF). DDM is hydrogenated to cis-tetrol over a Pt/SiO2 catalyst, while DDG is hydrogenated to trans-tetrol. Formation of DDF erases the stereocenter at the C2 position of lgol, and hydrogenation of DDF produces a nearly 1 : 1 mixture of cis- and trans-tetrol. This catalytic approach to produce tetrol from biomass opens the door to sustainable chemicals derived from tetrol.


 Please wait while we load your content...
Please wait while we load your content...