Water-stable fluorinated metal–organic frameworks (F-MOFs) with hydrophobic properties as efficient and highly active heterogeneous catalysts in aqueous solution†
Abstract
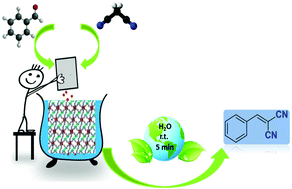
Two new fluorinated metal–organic frameworks [Zn2(hfipbb)2(4-bpdh)]·0.5DMF (TMU-55) and [Zn2(hfipbb)2(4-bpdb)]·2DMF (HTMU-55) have been solvothermally synthesized by the reaction of two linear N-containing ligands as pillars (4-bpdb = 1,4-bis(4-pyridyl)-2,3-diaza-1,3-butadiene and 4-bpdh = 2,5-bis(4-pyridyl)-3,4-diaza-2,4-hexadiene), and a flexible V-shaped fluorinated linker, H2hfipbb = 4,4′-(hexafluoroisopropylidene) bis(benzoic acid). The two compounds were characterized by different techniques such as X-ray crystallography, powder X-ray diffraction (PXRD), infrared spectroscopy (IR), contact angle (CA), thermogravimetry (TGA), field emission scanning electron microscopy (FE-SEM), inductively coupled plasma (ICP), and Brunauer–Emmett–Teller (BET) surface area analysis. Both compounds are structurally similar and exhibit three dimensional (3D) coordination frameworks with hydrophobic properties. The catalytic activity of these isoreticular F-MOFs as efficient base heterogeneous catalysts toward the Knoevenagel condensation reaction was tested and compared to each other. TMU-55 and HTMU-55 exhibited good catalytic activity (with a minimum amount of catalyst, 2.5 mg (0.5 mol%), and the shortest time, 5 min) in water as a green solvent with excellent conversions. Also, the results were considerably higher in comparison with most of the values given in the literature for this reaction at ambient temperature. The remarkable catalytic performance of TMU-55 and HTMU-55 in water can be attributed to the presence of hydrophobic fluoro groups near the basic reaction center in the F-MOFs. These catalysts maintain their crystalline frameworks after the reaction and are easily recovered and reused at least for three cycles without significant loss in their catalytic activity. Furthermore, the conversion of benzaldehyde can be kept over 95% while the selectivity of the Knoevenagel reaction is kept at 100%.


 Please wait while we load your content...
Please wait while we load your content...