Development of a H3PW12O40/CeO2 catalyst for bulk ring-opening polymerization of a cyclic carbonate†
Abstract
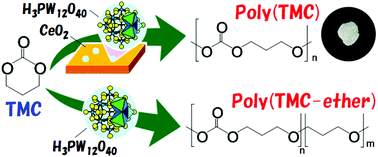
A new reaction system involving a heterogeneous H3PW12O40/CeO2 catalyst and methyl iodide initiator was developed for bulk ring-opening polymerization (ROP) of trimethylene carbonate (TMC). Combination of a Brønsted acid (H3PW12O40) and Lewis base (CeO2) had a synergic effect on a well-controlled ROP without decarboxylation, which gave poly(TMC) of molecular weight (Mn) 30 000 and Polydispersity Index (PDI) of 1.80 at 60 °C and 24 h. Fourier transform infrared (FTIR) spectroscopy elucidated a reaction mechanism in which H3PW12O40 promoted the initiation reaction and CeO2 activated TMC, and the interface of these two components was an active site for ROP. The catalyst was removed readily by filtrating a dimethyl carbonate solution of poly(TMC), which was confirmed by inductively coupled plasma-atomic emission spectroscopy. In addition, various kinds of biomass-derived poly(aliphatic carbonate)s were synthesized and thermal properties were investigated by differential scanning calorimetry and thermogravimetry-differential thermal analysis. In particular, pyrolysis-gas chromatography mass spectrometry of these polymers revealed that the degradation mechanism was highly dependent upon a small amount of ether linkages and a pendant methyl group.


 Please wait while we load your content...
Please wait while we load your content...