A versatile biosynthetic approach to amide bond formation†
Abstract
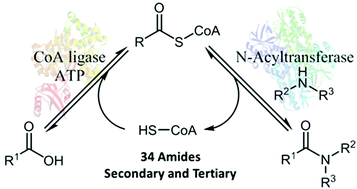
The development of versatile and sustainable catalytic strategies for amide bond formation is a major objective for the pharmaceutical sector and the wider chemical industry. Herein, we report a biocatalytic approach to amide synthesis which exploits the diversity of Nature's amide bond forming enzymes, N-acyltransferases (NATs) and CoA ligases (CLs). By selecting combinations of NATs and CLs with desired substrate profiles, non-natural biocatalytic pathways can be built in a predictable fashion to allow access to structurally diverse secondary and tertiary amides in high yield using stoichiometric ratios of carboxylic acid and amine coupling partners. Transformations can be performed in vitro using isolated enzymes, or in vivo where reactions rely solely on cofactors generated by the cell. The utility of these whole cell systems is showcased through the preparative scale synthesis of a key intermediate of Losmapimod (GW856553X), a selective p38-mitogen activated protein kinase inhibitor.
- This article is part of the themed collection: Celebrating our 2024 Prizewinners


 Please wait while we load your content...
Please wait while we load your content...