Ambient stable naphthalenediimide radical ions: synthesis by solvent-free, sonication, mechanical grinding or milling protocols†
Abstract
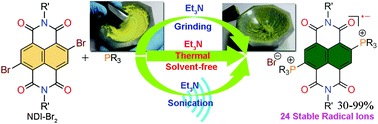
An efficient solvent-free synthetic method for the preparation of diphosphonium substituted naphthalenediimide (NDI) radical ions [NDI(PR3)2]˙+ Br− (R = alkyl/aryl) with excellent yields and ambient stability has been described. A diverse range of trialkyl- and triarylphosphines along with different groups at the axial-positions of the NDI were assessed to verify the efficacy of this new synthetic protocol. A library comprising 24 ambient stable radical ions was synthesized and isolated via solvent-free and conventional heating conditions. The reactions were found to be quite fast and could be completed within 10–20 minutes. In addition, we applied sonication to synthesize the NDI radical ions, when the phosphine reactants exist in the liquid state. Intriguingly, NDI radical ion formation was also observed even on manual grinding of the reactants in a mortar and pestle, although with poor yields. Furthermore, we also realized NDI radical ions in moderate yields by applying ball mill grinding. In addition, we have made an attempt to unearth the most plausible reaction mechanism for these reactions. Importantly, this is the first instance whereby synthetic radical ion chemistry has been brought under the realm of green synthesis. The other significant aspect of our findings is the eco-friendly formation of two consecutive C–P bonds and the in situ one-electron transfer, which can have vital ramifications in organophosphorus and electron transfer chemistry.


 Please wait while we load your content...
Please wait while we load your content...