Catalytic conversion of biomass derivatives to lactic acid with increased selectivity in an aqueous tin(ii) chloride/choline chloride system†
Abstract
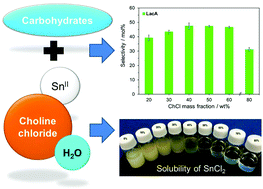
Catalytic conversion of biomass-derived carbohydrates to lactic acid (LacA) over Sn-based catalysts always shows a low selectivity (<30%) in an aqueous system, especially when SnCl2 is used as the catalyst. In this study, the catalytic activity as well as the selectivity of SnCl2 for the conversion of trioses, monosaccharides and disaccharides into LacA in the aqueous phase reaction system was found to be increased by the addition of choline chloride (ChCl). As the concentration of ChCl was 40–50%, the reaction selectivities towards LacA reached 40–77% under moderate reaction conditions (155–190 °C for 0.5–1.5 h). It is attributed to the formation of the SnCl2/ChCl complex in the H2O–ChCl system, which was indicated by (1) the pH of the solution changing to the acidic state (i.e. ∼3), (2) the SnCl2 completely dissolving in the ChCl aqueous solution, and (3) the single chemical shift in 119Sn NMR spectra. Such a SnCl2/ChCl complex served as a bifunctional acid catalyst to promote the hexose reaction pathway via [3 + 3] retro-aldol reaction into LacA. In contrast, LacA selectivity was only ∼7% in neat water under the same operational conditions since SnCl2 can be easily hydrolyzed into Sn(OH)Cl and HCl, resulting in the dehydration of hexoses into levulinic acid due to the strong Brønsted acidity of HCl. A kinetic study revealed that LacA yield was greatly affected by the reaction temperature and reached the maximum at 160–190 °C after 15–30 min. This study provides a new insight for the possible application of this H2O–ChCl system in other Lewis acid catalysis reactions by using SnCl2 as the catalyst.


 Please wait while we load your content...
Please wait while we load your content...