Unraveling the mechanism of the oxidation of glycerol to dicarboxylic acids over a sonochemically synthesized copper oxide catalyst†
Abstract
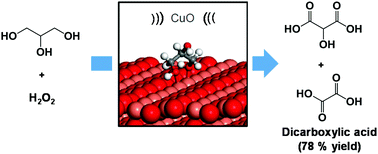
The utilization of low frequency ultrasound (US) offers a straightforward and powerful tool for the production of nanostructured materials, in particular for structurally stable, highly crystalline, and shape-controlled catalytic materials. Herein, we report an unconventional strategy for the synthesis of CuO nanoleaves within 5 min of US irradiation. The as-obtained CuO nanoleaves were found to be selective in the base-free aqueous oxidation of glycerol to dicarboxylic acids (78% yield of tartronic and oxalic acids), in the presence of hydrogen peroxide (H2O2) and under mild reaction conditions. Density Functional Theory (DFT) investigations revealed a synergy between the CuO catalyst and H2O2 in maintaining the structural integrity of the catalyst during the reaction, creating alternative efficient pathways for the selective formation of dicarboxylic acids. Isotope labeling experiments using H218O2 further confirmed that oxygen from hydrogen peroxide, not from CuO, was preferentially incorporated into the dicarboxylic acid, significantly preserving the monoclinic structure of the CuO catalyst.


 Please wait while we load your content...
Please wait while we load your content...