Towards environmentally friendlier Suzuki–Miyaura reactions with precursors of Pd-NHC (NHC = N-heterocyclic carbene) complexes†‡
Abstract
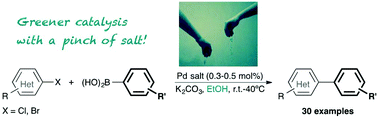
The preparation of [NHC·H][Pd(η3-R-allyl)Cl2] complexes is disclosed and represents a facile, atom-economical, environmentally friendly and rapid synthesis. These palladates are immediate synthetic precursors to the well-known [Pd(NHC)(η3-R-allyl)Cl] complexes. Their activation leading to catalytically relevant species has been studied in the Suzuki–Miyaura reaction. The need for an activation step prior to the catalysis was examined. The reaction scope showcases its ease and breadth in terms of functional group tolerance. Electron-donating and electron-withdrawing aryl chlorides and bromides were coupled effectively as well as heteroatom-containing and sterically hindered aryl halides. The catalytic reaction was conducted in ethanol with a weak and inexpensive inorganic base.


 Please wait while we load your content...
Please wait while we load your content...