Hf-based metal–organic frameworks as acid–base catalysts for the transformation of biomass-derived furanic compounds into chemicals†
Abstract
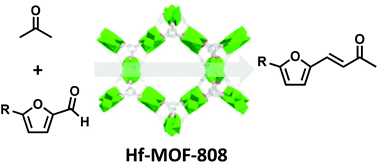
Hf-based metal–organic frameworks (MOFs) are reported here as heterogeneous catalysts for the highly selective and efficient cross-aldol condensation of biomass-derived furanic carbonyls with acetone under mild reaction conditions with near quantitative yields. NMR studies with isotopically labeled acetone confirm that acid–base pairs in the MOF framework promote the soft enolization of acetone through α-proton abstraction. The catalyst, Hf-MOF-808, can be recycled several times with only a minor decrease in catalytic activity, which could be regained by Soxhlet extraction. Furthermore, Hf-MOF-808 maintains activity in the presence of frequent contaminants in biomass-based molecules such as water and acids, unlike traditional base catalysts. The generality of the procedure was shown by accomplishing the transformation with aromatic and aliphatic aldehydes with acetone as the enolizable component to yield the corresponding α,β-unsaturated methyl ketones which are versatile synthons in fine chemistry. Hf-MOF-808 could also be used in the one-pot synthesis of allylic alcohols by the sequential aldol condensation reaction to yield the α,β-unsaturated methyl ketone and the subsequent Meerwein–Ponndorf–Verley reduction of the carbonyl by a simple solvent exchange from acetone to isopropyl alcohol. Furthermore, Hf-MOF-808 was decorated with palladium particles and the resultant material could be used in the one-pot aldol condensation and subsequent highly selective double bond reduction.


 Please wait while we load your content...
Please wait while we load your content...