Switchable oil–water phase separation of ionic liquid-based microemulsions by CO2†
Abstract
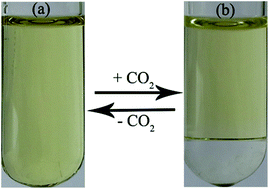
Phase separation of microemulsions plays an important role in many applications such as oil recovery, nanomaterials synthesis, and chemical reactions. However, reversible switching from ionic liquid-based microemulsions to complete oil–water phase separation has not been reported so far. In this work, we developed a novel class of stimuli-responsive microemulsions composed of CO2-responsive ionic liquids, n-pentanol and water. The microstructures and phase behavior of the microemulsion systems before and after the bubbling of CO2 were investigated by electrical conductivity, dynamic light scattering, small-angle X-ray scattering, cryogenic transmission electron microscopy, and optical microscopy. It was found that these microemulsions could be reversibly switched from W/O monophase to complete oil–water phase separation upon alternate bubbling and removal of CO2 at atmospheric pressure. Furthermore, 13C NMR spectroscopy was used to understand the CO2-driven reversible phase separation of the microemulsions. The results suggest that the mechanism behind the reversible phase separation involved the reversible formation of bicarbonate and carbamate from the reaction between CO2 and the anions of the ionic liquids in the presence of water, which resulted in the increase of ionic strength (or vice versa) in the mixture. Using the microemulsions as microreactors, the phase separation protocol was applied in the Knoevenagel reaction for an efficient coupling of a chemical reaction, product separation, and recycling of the microemulsions.


 Please wait while we load your content...
Please wait while we load your content...