Hydrogenolysis of methyl glycolate to ethanol over a Pt–Cu/SiO2 single-atom alloy catalyst: a further step from cellulose to ethanol†
Abstract
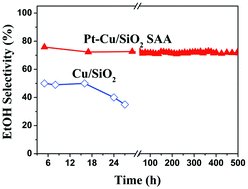
Cellulosic ethanol can be produced via a chemocatalytic approach in which cellulose is first converted into methyl glycolate (MG) in methanol using a W-based catalyst under an oxygen atmosphere, and the formed MG is then hydrogenated to ethanol over a supported copper catalyst. Aiming at enhancing the ethanol selectivity for this two-step approach, we developed a 0.1Pt–Cu/SiO2 single-atom alloy catalyst in the present study, where Pt was isolated as single atoms, and formed a Pt–Cu alloy phase. The characterizations of the catalyst via in situ XRD, FTIR, CO-adsorbed DRIFTS, N2O chemisorption, and XPS revealed that the introduction of 0.1 wt% Pt into the Cu/SiO2 catalyst resulted in an improvement in the Cu dispersion and an increase in the Cu+/Cu0 ratio. Moreover, Pt single atoms promoted the activation of hydrogen. As a consequence, the catalytic activity and selectivity of ethanol were enhanced; the maximum selectivity of ethanol (76.7%) was obtained at 503 K. Over 700 h on stream, the catalyst did not show any deactivation, demonstrating a promising stability.


 Please wait while we load your content...
Please wait while we load your content...