Valorization of 2,5-furandicarboxylic acid. Diels–Alder reactions with benzyne†
Abstract
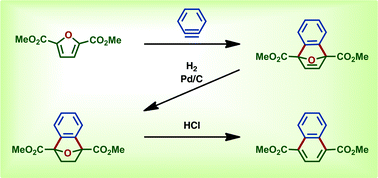
Biomass-derived 2,5-furandicarboxylic acid was valorized by conversion to 1,4-naphthalenedicarboxylic acid via benzyne-cycloaddition and reductive aromatization in 66% overall yield (four steps). Two novel bicyclic intermediates were isolated in 80% and 98% yield. These advances diversify the potential end uses of renewable terephalic acid analogs and other furanics available from cellulose biorefinery.


 Please wait while we load your content...
Please wait while we load your content...