Template-free and room temperature synthesis of hierarchical porous zeolitic imidazolate framework nanoparticles and their dye and CO2 sorption†
Abstract
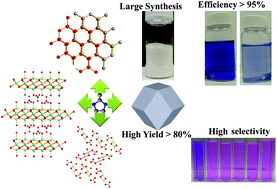
Hierarchical porous zeolitic imidazolate framework ZIF-8 nanoparticles have been synthesized using zinc nitrate, 2-methylimidazole (Hmim), and sodium hydroxide. Zinc hydroxide nitrate nanosheets were formed as intermediates that further transformed to hierarchical porous ZIF-8 after the addition of Hmim. These intermediates serve as in situ sacrificial templates and promote the formation of hierarchical porous ZIF-8 without the need for any other templates. The surface area and mesoporosity of the materials can be tuned by adjusting the concentration of NaOH. This method offers a fast and template-free approach for the synthesis of pure hierarchical porous ZIF-8 at room temperature with tunable porosity. The approach has been applied to synthesize two-dimensional ZIF leaf-like materials, ZIF-L. The synthesis of ZIF-8 and ZIF-L can be scaled up with high yields (>80%). The resulting ZIF-8 and ZIF-L materials show very good CO2 sorption properties. ZIF-8 nanoparticles show fast (<5 min), selective, and high efficiency (>95%) uptake of methyl blue in aqueous solution both without and in the presence of other dyes. The results open a new avenue for the understanding of the self-assembly and the formation of hierarchical porous ZIFs.


 Please wait while we load your content...
Please wait while we load your content...