A palladium-catalyzed dehydrative N-benzylation/C–H benzylation cascade of 2-morpholinoanilines on water†
Abstract
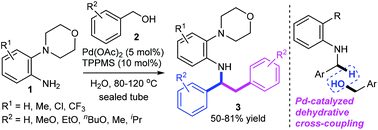
A strategy for the palladium-catalyzed dehydrative tandem benzylation of 2-morpholinoanilines with benzyl alcohols has been developed. This cascade reaction is devised as a straightforward and efficient synthetic route for N-(1,2-diphenylethyl)-2-morpholinoanilines in moderate to good yields (50–81%). The dehydrative sp3 C–H bond benzylation proceeds chemoselectively at the benzylic position of N-benzyl-2-morpholinoaniline to form a new C(sp3)–C (sp3) bond. KIE experiments show that C–H bond activation is involved in the rate-determining step (KIE = 2.7). A Hammett study of 2-morpholinoanilines gives a negative ρ value, suggesting that there is a build-up of positive charge in the transition state. A “on water” protocol, which affords the corresponding desired products with water as the sole co-product, can be achieved under mild reaction conditions without the need for bases or other additives in an atom-economical process.


 Please wait while we load your content...
Please wait while we load your content...