Extraction and recovery of Co2+ ions from spent lithium-ion batteries using hierarchical mesosponge γ-Al2O3 monolith extractors†
Abstract
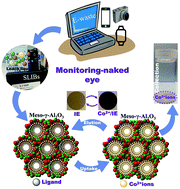
As visual extraction, detection, and recovery of Co2+ ions from spent lithium-ion batteries (SLIBs) via a one-step process becomes a new attractive, simple route for the management of urban electronic waste (e-waste), it will in turn lead ideally to the exploitation of accumulated e-waste and protection of the green environment. The Co2+ ion-capture system was achieved by selectively binding with synthesized chelating agents, namely, (E)-4-[(2-mercaptophenyl)diazenyl]-2-nitrosonaphthalen-1-ol (MPDN) and (E)-5-[(1,3,4-thiadiazol-2-yl)diazenyl]benzene-1,3-diol (TDDB), at controlled pH. The dense arrangement of MPDN and TDDB into microscopic, mesospongy γ-Al2O3 monoliths enabled the design of a solid/sponge Co2+ ion extractor (IE) from the SLIB leach liquor. Our recycling process of Co2+ ions from SLIBs showed evidence of (i) Co2+ ion waste management, (ii) low-cost collection/recovery of Co2+ ions, (iii) sensitive and selective extraction of ultra-trace Co2+ ion, and (iv) reduction of e-waste volume through multiple reusability or recyclability. Furthermore, our sponge IE design with large surface area-to-volume ratios, macro/mesopores, and grooves along the micrometric, hierarchical monolith structures results in a facile, naked eye monitoring of ultra-trace Co2+ ion collection/binding to a detection limit of approximately 3.05 × 10−8 M during multifunction extraction steps from SLIBs. Our results also showed evidence of the extraction of Co2+ ions (196 mg g−1) from SLIBs by a one-step process. This finding provides a basis for the control of multifunction processes (i.e., extraction, detection, and recovery) and the high performance needed for selective extraction and recovery of Co2+ ions from SLIBs in a one-step process.


 Please wait while we load your content...
Please wait while we load your content...