Key properties of Ni–MgO–CeO2, Ni–MgO–ZrO2, and Ni–MgO–Ce(1−x)Zr(x)O2 catalysts for the reforming of methane with carbon dioxide†
Abstract
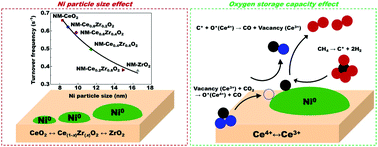
CeO2, ZrO2, and CeO2–ZrO2 supported on MgO-promoted Ni catalysts are developed and applied to the reforming of methane with carbon dioxide. The catalysts are prepared by the one-step co-precipitation/aging method and characterized through various techniques such as X-ray diffraction, Brunauer–Emmet–Teller measurements, pore distribution, X-ray photoelectron spectroscopy, X-ray absorption near edge spectroscopy, extended X-ray absorption fine structure analysis, H2-temperature programmed reduction, H2-chemisorption, Raman analysis, thermogravimetry analysis, and pulse H2–CO2 reactions. Ni–MgO–CeO2 shows the smallest Ni particle size and the particle size decreases with increasing ZrO2 content. Ni–MgO–Ce0.6Zr0.4O2 exhibits the largest oxygen storage capacity among the prepared catalysts. The size of the Ni particles and the oxygen storage capacity are found to be the primary and secondary key factors that influence the catalytic performance, respectively. The turnover frequency is dependent on the size of the Ni particles, but the catalytic performance is affected by the number of Ni active sites, which is estimated from the reduction degree and Ni particle size. Overall, the Ni–MgO–Ce0.8Zr0.2O2 catalyst shows the best performance owing to the high reduction degree and small Ni particle size.


 Please wait while we load your content...
Please wait while we load your content...