Ni3P as a high-performance catalytic phase for the hydrodeoxygenation of phenolic compounds†
Abstract
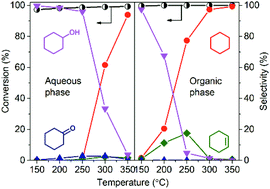
The catalytic performance of an unsupported Ni2P in the aqueous phase hydrodeoxygenation (HDO) of phenol was investigated. It was found that the unsupported Ni2P was water-sensitive, being transformed stepwise, first to an amorphous phase and then to Ni5P2 and Ni12P5, and finally to Ni3P in the presence of water at elevated temperatures. Nonetheless, the generated Ni3P phase exhibited extraordinary hydrogenation activity at low temperatures and high HDO activity at high temperatures. The unsupported Ni3P was more active for the hydrogenation of the aromatic ring in the phenol molecule than Pd/SiO2 (1.0 wt%). The unsupported Ni3P was catalytically active and stable in phenol HDO in both the aqueous phase and the organic phase. In addition to phenol, catechol and o-cresol were investigated in the HDO catalyzed by the unsupported Ni3P in both aqueous solution and decalin solution. The HDO reactivity decreased in the order of phenol > catechol > o-cresol in the aqueous phase, and in the order of phenol > o-cresol in the organic phase (catechol is insoluble in decalin). In the oil phase HDOs of phenol and o-cresol, the unsupported Ni3P exhibited superior hydrogenation activity to that of the unsupported Ni2P at low temperatures.


 Please wait while we load your content...
Please wait while we load your content...