Structural analysis of humins formed in the Brønsted acid catalyzed dehydration of fructose†
Abstract
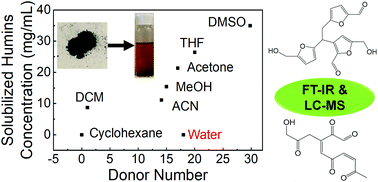
We use infrared (IR) spectroscopy, gel permeation chromatography (GPC), and liquid chromatography-mass spectrometry (LC-MS) in multistage dissolution experiments in various solvents to investigate the solubility and molecular structure of humins formed during fructose dehydration. We demonstrate that the soluble fraction of humins correlates positively with the donor number of solvents resulting in significant dissolution at room temperature in solvents with high donor number. Most of the solubilized humins fragments have relatively low molecular weight (Mw), and the same species are present in different quantities as the residual solid is repeatedly dissolved in the same solvent. In contrast to the common belief of humins consisting of polymers of large Mw, we postulate for the first time that they are spatially and chemically heterogeneous and consist of insoluble macromolecules and small soluble species that are weakly associated within the structure. The solubility profiles of dissolved species in acetonitrile and methanol are different in terms of Mw but possess similar IR spectra, indicative of similar functional groups in dissolved species. Furthermore, we hypothesize that the identified humins fragments form through aldol condensation between 5-hydroxymethylfurfural and its hydrated products and through condensation of furanic species.


 Please wait while we load your content...
Please wait while we load your content...