Synthesis of highly functional carbamates through ring-opening of cyclic carbonates with unprotected α-amino acids in water†
Abstract
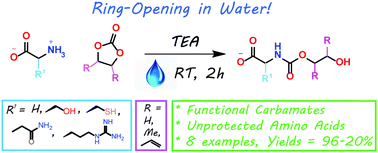
The present work shows that it is possible to ring-open cyclic carbonates with unprotected amino acids in water. Fine tuning of the reaction parameters made it possible to suppress the degree of hydrolysis in relation to aminolysis. This enabled the synthesis of functionally dense carbamates containing alkenes, carboxylic acids, alcohols and thiols after short reaction times at room temperature. When Glycine was used as the nucleophile in the ring-opening with four different five membered cyclic carbonates, containing a plethora of functional groups, the corresponding carbamates could be obtained in excellent yields (>90%) without the need for any further purification. Furthermore, the orthogonality of the transformation was explored through ring-opening of divinylenecarbonate with unprotected amino acids equipped with nucleophilic side chains, such as serine and cysteine. In these cases the reaction selectively produced the desired carbamate, in 70 and 50% yield respectively. The synthetic design provides an inexpensive and scalable protocol towards highly functionalized building blocks that are envisioned to find applications in both the small and macromolecular arena.


 Please wait while we load your content...
Please wait while we load your content...