Carbonated water for the separation of carboxylic compounds: a chromatography approach†
Abstract
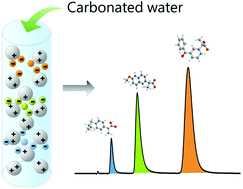
Green chemistry seeks to reduce the environmental impact of chemical products and processes through waste reduction and the use of safer solvents and substances. Chromatographic separation in particular generates significant amounts of harmful organic solvents, acids, bases, and salts, as waste by-products. CO2-modified solvents have been reported as promising routes to the development of greener analytical methodologies, but normally with stationary phases that are inert to the chemical effects of the CO2 modifier. We now report the impact of pH and dissolved CO2 mobile phase concentration on separations that utilize primary, secondary and tertiary amine functionalized silica particles. Ibuprofen, naproxen and ketoprofen, chosen as examples of pharmaceutical analytes containing carboxylic acid groups, have now been separated with CO2-modified (carbonated) water. Chromatographic retention, selectivity and zeta potential are utilized to demonstrate a dynamic ion exchange mechanism involving both protonation and deprotonation of stationary phase and analyte functional groups. Primary and secondary amine functionalized columns retain the test carboxylic acid compounds more strongly (tR ≥ 15 min), suggesting that they are beneficial for separations requiring higher retention. The use of CO2-modified mobile phases has the potential to significantly reduce both solvent and permanent acid use in some chromatographic separations.


 Please wait while we load your content...
Please wait while we load your content...