Characterization of pepsin from rabbit gastric extract, its action on β-casein and the effects of lipids on proteolysis
Abstract
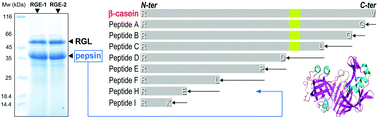
Rabbit gastric extract (RGE) is a source of gastric enzymes for in vitro digestion studies. While its gastric lipase activity has been characterized and compared to other lipases, its pepsin activity has not been studied. We measured pepsin activity in RGE using both hemoglobin and azocoll as substrates, and identified the protein separated by SDS-PAGE as a type II-4 mature pepsin of 328 amino acid residues using Edman sequencing, LC-MS/MS analysis and intact mass measurement. As a proof-of-concept that RGE was suitable for in vitro digestion of both proteins and lipids, it was used for studying the proteolysis of β-casein under conditions mimicking the early stages of intragastric digestion. β-Casein was displayed either in solution or at the surface of a β-casein-stabilized rapeseed oil emulsion to investigate the impact of lipids and lipolysis on proteolysis. Proteolysis of β-casein was quantified based on the kinetics of β-casein disappearance, the identification of various peptides generated upon digestion and their variation with time. The results obtained with RGE were highly similar to those obtained with equivalent amounts of porcine pepsin used as a reference standard. Digestion of β-casein was slower when it was displayed at the oil–water interface and some degradation peptides were transiently observed at higher levels and for a longer time than with β-casein in solution, or accumulated upon digestion. N-terminal sequencing of the main isolated peptides revealed a sequential action of pepsin starting from the hydrophobic C-terminal end of β-casein, which was impaired by the interaction of β-casein with lipids.


 Please wait while we load your content...
Please wait while we load your content...